William R. (Bill) Strohl Biography Overview
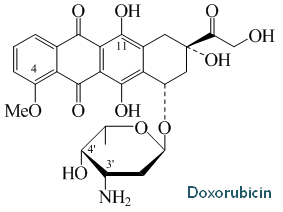
Bill has over 35 years of experience in biopharmaceutical research, starting with a 17-year academic career in academia at The Ohio State University which he focused on the biosynthesis and molecular biology of doxorubicin biosynthesis and the use of natural product biosynthesis genes to make novel natural products (examples of papers include references 1-5, below).
From 1980 to 1997, Dr. Strohl was promoted from Assistant to Full Professor in the Department of Microbiology and the Program of Biochemistry at The Ohio State University. There he pursued the molecular biology and biochemistry of polyketide biosynthesis pathways, particularly doxorubicin, in actinomycetes, and the physiology of E. coli in computer-controlled high cell density fermentations. As part of these efforts, Bill and his students used natural product biosynthesis genes to make novel natural products, which was at the cutting edge of natural products research in the 1980s and early 1990s (examples of papers include references 1-5, below).
Additionally, with his students, Bill got involved in fermentation engineering (refs 6-8), which brought him closer to many aspects of the biopharma industry. As such, most of Bill’s 17 Ph.D. student graduates were recruited into the biopharma industry then or later. In 1997, Bill was recruited to join the Natural Products group at Merck and Co., which at the time was one of the premier natural products organizations in the world (9).
During his time in Merck Natural Products, Bill and his team built a substantial natural product library which, in part, today resides at Natural Products Discovery Institute (NPDI) in Doylestown, PA (http://www.npdi-us.org/), and Bill got involved in natural products for infectious diseases and other therapeutic areas (10-11). Additionally, Bill’s team incorporated recombinant techniques to make novel natural products libraries (12-13), but these efforts were cut short by changes in the industry at the time. At that point in the history of the pharmaceutical industry, the discovery of natural products as drugs was beginning to compete head-to-head with high throughput screening of enormous synthetic organic chemical libraries (10). Thus, in the late 1990s and early 2000s, natural products programs across the industry, as well as at Merck, were either shrunk substantially or eliminated altogether.
With the shrinking of natural products efforts at Merck, came a new and exciting opportunity for Bill, starting in 2000, to get involved in biologics. At that time, Bill was asked to initiate a Microbial Vaccines department, and later, to start and lead the Biologics discovery efforts, where he was involved in two biotechnology company acquisitions and multiple licenses to improve the discovery capabilities. From that time to the present, Bill has devoted his career to making and improving therapeutic proteins, most of which have been monoclonal antibodies or derivatives thereof. References for a few of my more recent papers in this area (14-20) are enclosed below.
Bill left Merck in 2008 and joined Centocor, which was owned by Johnson & Johnson, as head of antibody drug discovery. Within a bit more than a year of Bill joining Centocor, it became part of the Janssen R&D, and as part of that change, Bill was asked to head up all of biologics discovery. As head of Biologics Research, the discovery arm of Janssen BioTherapeutics, Bill led the doubling in both the size and output of the biologics, and helped to significantly improve antibody engineering of the clinical development candidates. In 2013, Bill was appointed VP and head of the Biotechnology Center of Excellence (later renamed Janssen BioTherapeutics). As VP and Head, Janssen BioTherapeutics, Janssen R&D, Bill oversaw biologics discovery, early development, and technology development. In that role, Bill also served as a member of the Janssen R&D Senior Leadership Team under the direction of Dr. William Hait.
As part of Bill’s biotechnology leadership role, he initiated new areas of research in gene and cell therapy to augment the long-standing traditional efforts in antibodies and protein therapeutics at J&J. Over his time at J&J, Dr. Strohl and his teams placed more than 30 highly innovative, novel biologics into development, many of which are in clinical development today (see table).
Upon his retirement from Janssen R&D in August, 2016, William (Bill) Strohl, Ph.D. founded BiStro Biotech Consulting LLC, a consulting company designed to help biotechnology companies grow and expand their programs and capabilities.
References:
1. Bartel, P.L., C.-b. Zhu, J.L. Lampel, D.C. Dosch, N.C. Connors, W.R. Strohl, J.M. Beale, Jr., and
H.G. Floss. 1990. Biosynthesis of anthraquinones by interspecies cloning of actinorhodin
biosynthesis genes in streptomycetes: Clarification of actinorhodin gene functions. J.
Bacteriol. 172:4816-4826.
2. Dickens, M.L., J. Ye, and W.R. Strohl. 1995. Analysis of clustered genes encoding both early
and late steps in daunomycin biosynthesis by Streptomyces sp. strain C5. J. Bacteriol.
177:536-543.
3. Dickens, M.L., N.D. Priestley, and W.R. Strohl. 1997. In vitro and in vivo bioconversion of
ε-rhodomycinone glycoside to doxorubicin: Functions of DauK, DauP, and DoxA. J. Bacteriol.
179:2641-2650.
4. Strohl, W.R. 1997. Industrial antibiotics: Today and the future, pp. 1-47. In W.R. Strohl (ed.),
Biotechnology of Antibiotics, 2nd Edition. Marcel Dekker Publishers, New York.
5. Walczak, R.J., M.L. Dickens, N.D. Priestley, and W.R. Strohl. 1999. Purification, properties
and characterization of recombinant Streptomyces sp. strain C5 DoxA, a cytochrome P450
catalyzing multiple steps in doxorubicin biosynthesis. J. Bacteriol. 181:298-304.
6. Kleman, G.L., J. Chalmers, G.W. Luli, and W.R. Strohl. 1991. Predictive and feedback
control provides constant glucose concentration in fed-batch fermentations. Appl.
Environ. Microbiol. 57:918-923.
7. Kleman, G.L., and W.R. Strohl. 1992. High cell density and high-productivity microbial
fermentation. Curr. Opin. Biotechnol. 3:93-98.
8. Kleman, G.L., and W.R. Strohl. 1994. Developments in high cell density and high
productivity microbial fermentation. Curr. Opin. Biotechnol. 5:180-186.
9. Strohl, W.R., H.B. Woodruff, R.L. Monaghan, D. Hendlin, S. Mochales, A.L. Demain, and
J. Liesch. 2001. The history of natural products research at Merck and Co., Inc. Soc.
Industr. Microbiol. News 51: 5-19.
10. Strohl, W.R. 2000. The role of natural products in a modern drug discovery program.
Invited editorial. Drug Disc. Today 5:39-41.
11. Strohl, W.R. 2001. Biochemical engineering of natural product biosynthesis pathways.
Metab. Engn. 3: 4-14.
12. An, Z., and W.R. Strohl. 2003. Genomics in novel natural products generation. In: Microbial
Genomics and Drug Discovery, S.J. Projan and T. Dougherty, Eds. Marcel Dekker,
pp. 221-238.
13. An, Z., G. Harris, D. Zink, R. Giacobbe, R. Sangari, P. Lu, J. Greene, G. Bills, C. Meyers,
S. Smith, V. Svetnik, B. Gunter, A. Liaw, P. Masurekar, J. Liesch, S. Gould, and
W.R. Strohl. 2004. Expression of cosmid-size DNA of slow-growing fungi in Aspergillus
nidulans for secondary metabolite screening. In Z. An, ed. Handbook of Industrial Mycology,
pp. 167-187. Marcel Dekker, New York, NY.
14. Su, B., R. Hrin, B.R. Harvey, Y-J. Wang, R.E. Ernst, R.A. Hampton, M.D. Miller, W.R.
Strohl, Z. An and D.L. Montgomery. 2007. Automated high-throughput purification of
antibody fragments to facilitate evaluation in functional and kinetic based assays.
J. Immunol. Methods 322:94-103.
15. Strohl, W.R. 2009. Therapeutic Monoclonal Antibodies - Past, Present, and Future.
In: An, Zhiqiang, pp. 3-50. Therapeutic Antibodies, from Bench to Clinic.
John Wiley and Sons.
16. Strohl, W.R. 2009. Optimization of Fc-Mediated Effector Functions of Monoclonal
Antibodies. Curr. Opin. Biotechnol. 20:685-691.
17. Glantschnig, H., R. Hampton, P. Lu, J.Z. Zhao, S. Vitelli, L. Huang, P. Haytko, T. Cusick,
C. Ireland, S. Jarantow, R.Ernst, N.Wei, P. Nantermet, K. Scott, J. Fisher, F. Talamo,
L. Orsatti, A. Reszka, P. Sandhu, D. Kimmel, O. Flores, W. Strohl, Z. An, F. Wang.
2010. Generation of fully human monoclonal antibodies targeting dickkopf-1 (DKK1)
for the increase of bone mass. J. Biol. Chem 285:40135-40147.
18. Vafa, O., G.L. Gilliland, R.J. Brezski, B. Strake, T. Wilkinson, E.R. Lacy, B. Scallon,
A. Teplyakov, T. Malia, and W.R. Strohl. 2013. An engineered silent Fc variant of an
IgG eliminates all immune effector functions via structural perturbations. Methods
65: 114-126.
19. Redpath, N.T., Y. Xu, N.J. Wilson, A.E. Andrews, L.J. Fabri, M. Baca, P. Lu, C. Ireland,
R.E. Ernst, A. Woods, G. Forrest, Z. An, D.M. Zaller, W.R. Strohl, C.S. Luo,
P.E. Czabotar, T.P.J. Garrett, D.J. Hilton, A.D. Nash, J.-G. Zhang, and N.A. Nicola. 2013.
Production of a human neutralizing monoclonal antibody and its crystal structure in
complex with the ectodomain 3 of the interleukin-13 receptor. Biochem. J. 451, 165–175
20. Kinder, M., A.R. Greenplate, K. Grugan, G. Bannish, M. Perpetua, R.E. Jordan,
W.R. Strohl, and R.J. Brezski. 2013. Engineered protease-resistant antibodies with
selectable cell-killing functions. J. Biol. Chem. 288:30843-30854.
21. Zhang., N., H. Deng, X. Fan, A. Gonzalez, S. Zhang, R. Brezski, B.-K. Choi, M. Rycyzyn,
W. Strohl, R. Jordan, and Z. An. 2015. Dysfunctional antibodies in tumor
microenvironment associate with impaired anticancer immunity. Clin. Cancer Res.
21(23): 5380-5390.
22. Strohl, W.R. 2015. Fusion proteins for half-life extension of biologics as a strategy
to make biobetters. BioDrugs DOI 10.1007/s40259-015-0133-6. 25 pp.
Some fun history:
I am not the first scientist in my family, by a long shot. My uncle Russell Steere (picture below), my mother’s brother, was trained as a physicist but became a well-renown virologist in the 1960s. He was the inventor of the technology called “freeze-etching electron microscopy”, based on a seminal paper he published on that topic in 1957 as a new method to visualize the structure of tobacco mosaic virus (see figure below). Russell Steere had a huge influence on my decision to enter science, and I cannot thank him enough for that. But…, he was not the first scientist in our family, either.
While growing up, I learned from my Great Aunts about my great-grandfather, Joseph Beal Steere, who was the founder of the Museum of Natural History at the University of Michigan in Ann Arbor (https://lsa.umich.edu/ummnh/about/history.html). Joseph Beale Steere (sitting, in the middle with the rifle) was a great naturalist and ornithologist circa late 1800s who spent years in the Amazon basin, the Philippines, and Taiwan studying the natural habitats of hundreds of species of animals. Today, my home is graced with several of his books, many of his writings, and a stunning nautilus shell he collected on one of his trips.
They say the nut does not fall far from the tree. In my case, this appears to be true, as my older son, Joshua Strohl, is currently enrolled at Hofstra Northwell School of Medicine (http://medicine.hofstra.edu/) in collaboration with the Feinstein Institute (http://www.feinsteininstitute.org/) working on his Ph.D. in neurosciences.
Current Efforts:
Bill Strohl is currently working on several exciting projects that are keeping him very busy. In addition to consulting for two academic groups and ten industry organizations, Bill is writing several manuscripts, including a new review of Bispecific Antibodies, a review on the interaction of proteases and immunoglobulins, and a review (in collaboration with Michael Naso at Janssen) on gene therapy approaches using adeno-associated virus. Bill recently presented a 3-hour short course at PEGS-Boston on April 30, 2017, entitled “Target Selection for Biologics”. Bill also is in the process of developing a 12-hour course for Cambridge Health Institute (CHI) entitled: “Pharmaceutical Biotechnology Discovery – From Antibody Engineering to Gene Therapy” that will be presented at The Bioprocessing Summit, August 23-24, 2017 and then again at PEGS Europe in Lisbon, Portugal, during the week of November 13-17, 2017.
Additionally, Bill and his wife, Lila (pictured below), have begun two books – the first is a radical update of their highly successful book entitled: “Therapeutic Antibody Engineering: Current and Future Advances Driving the Strongest Growth Area in the Pharma Industry" (Woodhead Publishing [now Elsevier], 2012 ISBN 9781907568374). The second book, which should be the first of its kind anywhere, is preliminarily entitled: “The Art of Immunology”, and will feature stunning molecularly-based art by Lila Strohl with descriptions of the science illustrated by Bill Strohl. That book is intended to the first of a series of books focusing on the art of biotechnology subjects.
Bill Strohl Brief CV (1980-2016):
VP2, Biotechnology COE and Janssen BioTherapeutics, 2013-2016, and Sr. Director and VP1, Biologics Research, Centocor/Janssen, 2008-2013
o Rebuilt and drastically expanded efforts on antibody discovery
o Led discovery and early development organization with budget of >$170MM
o Led group that placed >30 biologics (mostly mAbs) into development in 8 years
o Led or significant involvement in 16 in-licensed technologies
o Wrote book entitled: Therapeutic Antibody Engineering, 2012 (Woodhead Publishing) which sold >1000 copies
o >50 publications and 16 patents while in industry (1997-2016)
Sr. Director and Exec. Director, Vaccines and Biologics, Merck, 2000-2008
o Led effort on microbial vaccine discovery
o Started phage display group and in-licensed library technologies
o Led group that placed 6 antibodies into development
o Led efforts to in-license 6 major technologies and acquire two biotech companies to build biologics capabilities
Sr. Director, Natural Products Microbiology, Merck 1997-2000
o Helped lead the building of a library of >50,000 microbial natural product extracts from a wide variety of microbial sources
Asst., Assoc., and Full Professor, The Ohio State University, Department of Microbiology and Program in Biochemistry, 1980-1997
o Elucidated most of the 34 step biochemical pathway for doxorubicin biosynthesis, cloned most of the
genes encoding the biosynthesis of doxorubicin, purified several enzymes of that pathway, and used
the genes to generate the FIRST novel polyketides by mixing and matching components of polyketide
synthases (1991)
o Funded for 16 straight years
o >80 publications and 2 patents as an academic researcher (1980-1997)